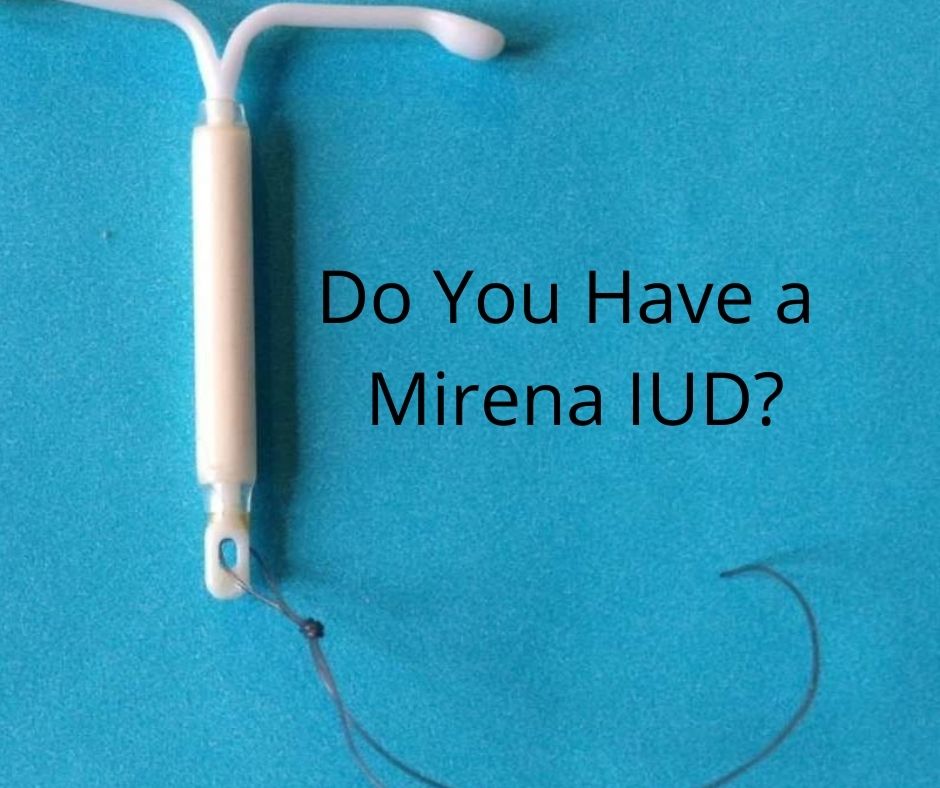
Be part of a Bayer-sponsored study on the extended use of Mirena. Pharmasite Research is participating in a Bayer-sponsored study on the extended use of the Mirena® IUD. The Mirena® intrauterine contraceptive has been approved as a safe and reliable method of contraception for up to 5 years by the Food & Drug Administration (FDA). The purpose of the study is to evaluate the effectiveness of Mirena® beyond 5 years, up to 8 years of use at preventing a pregnancy. Additionally, the study would assess menstrual blood loss, bleeding patterns and safety during the extended use. The results of this study may allow women to continue use of the Mirena® beyond 5 years, up to 8 years.
- You may be eligible to participate in this study if:
- You are a woman between the ages of 18 and 35
- You are currently using Mirena® for contraception OR contraception and heavy menstrual bleeding
- You have been using the same Mirena® for at least 4.5 years and are able to show documentation of the original date of insertion and indication for use.
- You are willing to use the study drug as your main form of contraception
- You are sexually active with a male partner who has not had a vasectomy
Qualified participants will receive:
- Medical exams
- Compensation for time and travel






